Friend of the cat I own an eye on. Thats what this is a salt.
 Solved Be Sure To Answer All Parts What Factors Qualify Chegg Com
Solved Be Sure To Answer All Parts What Factors Qualify Chegg Com
In practical terms all common ionic compounds except hydroxides and oxides are salts.
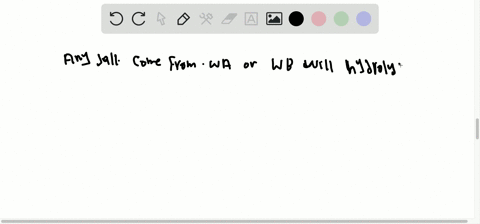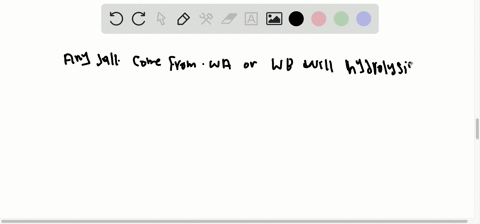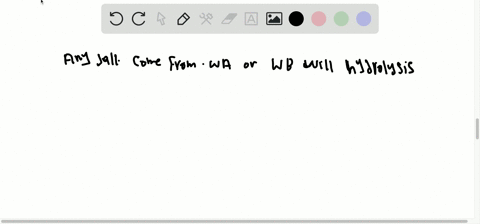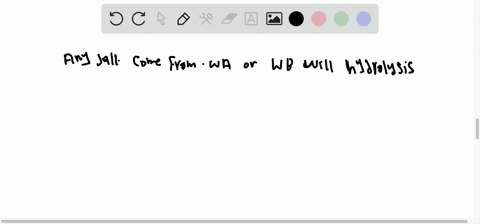
What factors qualify a compound as a salt. A salt is an ionic compound made up of the cation from a base and the anion from an acid. A salt is an ionic compound that can be formed by an acid-base reaction. Click card to see definition A homogenous mixture 2 or more substances consisting of a solvent and one or more solutes.
Salt factor is for conversion to free acid or base. In the answers below I have given an acid-base reaction that will form the compounds classified as salts. This is this hold.
Salts are composed of related numbers of cations positively charged ions and anions negatively charged ions so that the product is electrically neutral without a net charge. For example NaCl FeSO4 and AgF are all salts. CH4 NaF NaOH CaO BaSO4 HNO3 NH3 KBr - The factors are that salts are solids because they are a products of acid and base reaction and the salts must contain of a combination of an anion and a cation in which the cation isnt a H and anion isnt.
Next we have bilion. In chemistry a salt is a chemical compound consisting of an ionic assembly of cations and anions. Specify which of the following compounds are salts.
Move calcium cat ion and Occident and this fits the requirements of a salt before. 11What factors qualify a compound as a salt. -An ionic compound made up of the cation from a base and the anion from an acid can be qualified as a salt-Bases are proton H acceptors-Acids ionize in water to release hydrogen ions and a negitive ion other than hydroxide OH- NaF BaSO4 and KBr are salts-Salts are ionic compounds composed of a cation from a base and an anion from an acid.
If you have a positive metallic species bonded to some negative species it can generally be called a salt. What factors qualify a compound as a saltA Substances that ionize in water to produce H ionsB Substances that ionize in water to produce OH- ionsC An ionic compound made up of the cation from a base and the anion from an acid. Assigned purity HPLC Purity100 x 100- Sum of Non-HPLC Impurities x Salt Factor.
We have calcium oxide which is formed. There is some complication when you have large com. Its when its an eye on a compound.
BaSO 4 HNO 3 NH 3. The answer is a little bit subjective. At my place of worship oops I mean work we use the following equation for calculation of assigned purity.
This seems to work but my question has to do with COAs that have a test for the salt eg chloride for HCl. So feet which is form uh William and so feet. What factors qualify a compound as a salt.
Specify which of the following compounds are salts. A CH4 not a salt a molecular compound b NaF a salt NaOH HF NaF H2O.